French vaccine maker Valneva says the UK government has scrapped a deal for its Covid-19 vaccine.
The UK had about 100 million doses on order, after it increased its request by 40 million in February.
The company said in a statement that the UK government served notice over allegations of a breach of the agreement, which it “strenuously denies”.
Valneva jab is still being tested in trials.
Although regulators must be satisfied before the rollout of any vaccine, manufacturing at a site in West Lothian, Scotland, had already started.
The plant in Scotland employs about 250 people, including scientists and lab technicians.
In a statement on its website, Valneva said: “Valneva SE, a specialty vaccine company, today announced that it has received a termination notice from the UK Government (HMG) in relation to the Supply Agreement for its Covid-19 vaccine candidate, VLA2001.
“The contract provides HMG with the right to terminate. HMG has alleged that the company is in breach of its obligations under the supply agreement, but the company strenuously denies this.”

The firm said on Monday that results from its phase three trials were due later this year.
It added: “Valneva has worked tirelessly, and to its best efforts, on the collaboration with HMG including investing significant resources and effort to respond to HMG’s requests for variant-derived vaccines.”
The company hopes that, dependent on the results of its continuing trials and sign-off from the UK’s Medicines and Healthcare products Regulatory Agency (MHRA), initial approval could still be granted in 2021.
Its vaccine is expected to be given as two doses and contains a dead version of coronavirus that cannot cause disease, but should teach the body’s immune system how to fight it.
But given that it is not yet approved for use by UK regulators, it will not affect the current rollout of jabs.
Speaking to BBC Radio’s Good Morning Scotland, Scotland’s Health Secretary Humza Yousaf said: “We have enough supply even for a booster programme. I want to give absolute confidence to anyone listening that we have the supplies necessary to continue to vaccinate and particularly with a booster programme on the horizon.”
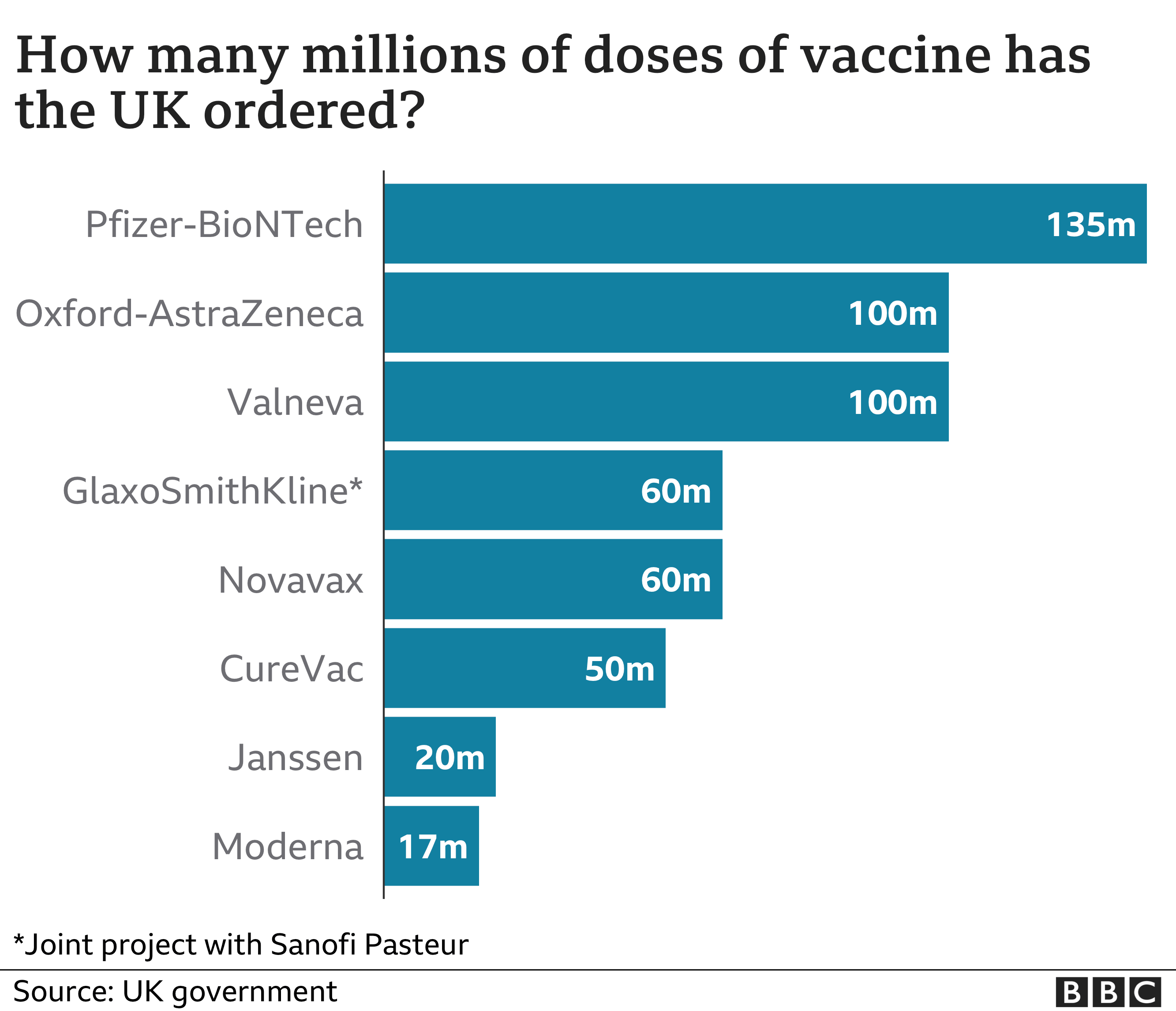
Mr Yousaf said that while the announcement would be a big set-back for the Livingston plant, he would speak to Valneva to discuss its future.
The Scottish Health Secretary added that he was waiting for further information from the UK government over Valneva alleged failure to meet the terms of its contract.
SNP MP Hannah Bardell, whose constituency contains the Valneva plant, said she was “incredibly disappointed” by the news.
She pledged she would be “working with Valneva – who have worked tirelessly on this vaccine – and will raise this urgently with the UK government”.
Analysis by Laura Goodwin, BBC Scotland
Valneva is a French company but this was billed as the vaccine made in Scotland.
I first visited the Livingston factory back in December 2020. At that point, the UK government had already pre-ordered 60 million doses of their inactivated whole virus vaccine.
The company has a background in making vaccines for other diseases and were hopeful their Covid-19 offering would have a good safety profile, perhaps making it suitable for children or those with compromised immune systems.
Given the early supply issues we experienced with some of the other vaccines, having one made here in the UK was also seen as a bonus.
The prime minister visited the factory earlier this year just as commercial production began and the order for Valneva vaccine was increased to 100 million doses. Two hundred extra staff were brought in and additional production space was built.
The Valneva vaccine was already being trialled as a potential booster and three weeks ago the company announced they had begun a rolling submission to regulators the MHRA with approval expected later this year.
So what now? The company say they were told over the weekend that the UK government is terminating the deal because of an alleged breach in the supply agreement, something Valneva deny. They say work will continue to gain approval, but they may now look to other countries.
Valneva said on Monday that it would also look to other potential customers to ensure that the vaccines can still be used in the fight against the pandemic.
French President Emmanuel Macron faced criticism earlier this year after failing to secure doses from the company, which has its headquarters near Nantes.
In March, he pledged that France’s vaccination roll-out would have caught up with the UK’s “in a few weeks” amid tensions over the vaccine supplies, although the country has since reached more than 90 million doses given.
The Department for Health did not immediately respond to the BBC’s request for comment.



No comments:
Post a Comment
Note: only a member of this blog may post a comment.