India has approved two new vaccines, expanding its programme amid fears of a third wave fuelled by Omicron.
The new vaccines – Serum Institute of India’s Covovax and Biological E’s Corbevax – have both been authorised for “restricted use in emergency situation”.
India has now approved eight vaccines, three of which have been developed in India.
The country has so far given more than 1.4 billion doses.
The government aimed to vaccinate all Indians by the end of this year but is falling short of that target. About 62% of eligible adults have been fully vaccinated and more than 90% have received at least one jab since the beginning of the drive in January.
Prime Minister Narendra Modi said that India would begin administering booster shots – or “precaution doses” as he called them – to healthcare and frontline workers, and those above 60 with comorbidities from 10 January.
He said India would also begin vaccinating 15-18-year-olds from 3 January.
India’s daily caseload is around 6,000, but cases of the Omicron variant – about 653 now – have been rising in several states, prompting night curfews and a slew of other restrictions.
It’s unclear still if the newly approved vaccines will be deployed for booster shots, or if the government will insist on the third shot being the same as the first two.
How do the new vaccines work?
Corbevax from Indian pharma company Biological E was developed in collaboration with US-based Dynavax and Baylor College of Medicine.
It is India’s first indigenously developed recombinant protein sub-unit vaccine, health minister Mansukh Mandaviya said. That is, it’s made up of the coronavirus’ “spike protein”, which the virus uses to latch on and enter human cells. When injected, this is expected to trigger an immune response in the body.
 IMAGE SOURCE,GETTY IMAGES
IMAGE SOURCE,GETTY IMAGESCovovax is a local version of the Novavax vaccine, and will be produced by the Serum Institute of India (SII), which is also manufacturing the Oxford-AstraZeneca jab, known locally as Covishield.
The vaccine was more than 90% effective in a late-stage US-based clinical trial, according to the company.
What vaccines had India approved before this?
India has already approved six other vaccines.
It’s currently using only three – Covishield, Covaxin by Indian firm Bharat Biotech and Russian-made Sputnik V – for its vaccination drive. Of these, Covishield accounts for over 90% of the doses given so far.
It also approved ZyCoV-D vaccine – the world’s first DNA vaccine against Covid – by Indian firm Cadilla, but it’s not available yet.
The federal government had also approved Johnson & Johnson’s single-dose vaccine, which was to be introduced in India through a supply agreement with Biological E; and it had authorised Indian pharma company Cipla to import the Moderna vaccine.
But it’s unclear when either of those will be available in India.
What do we know about these vaccines?
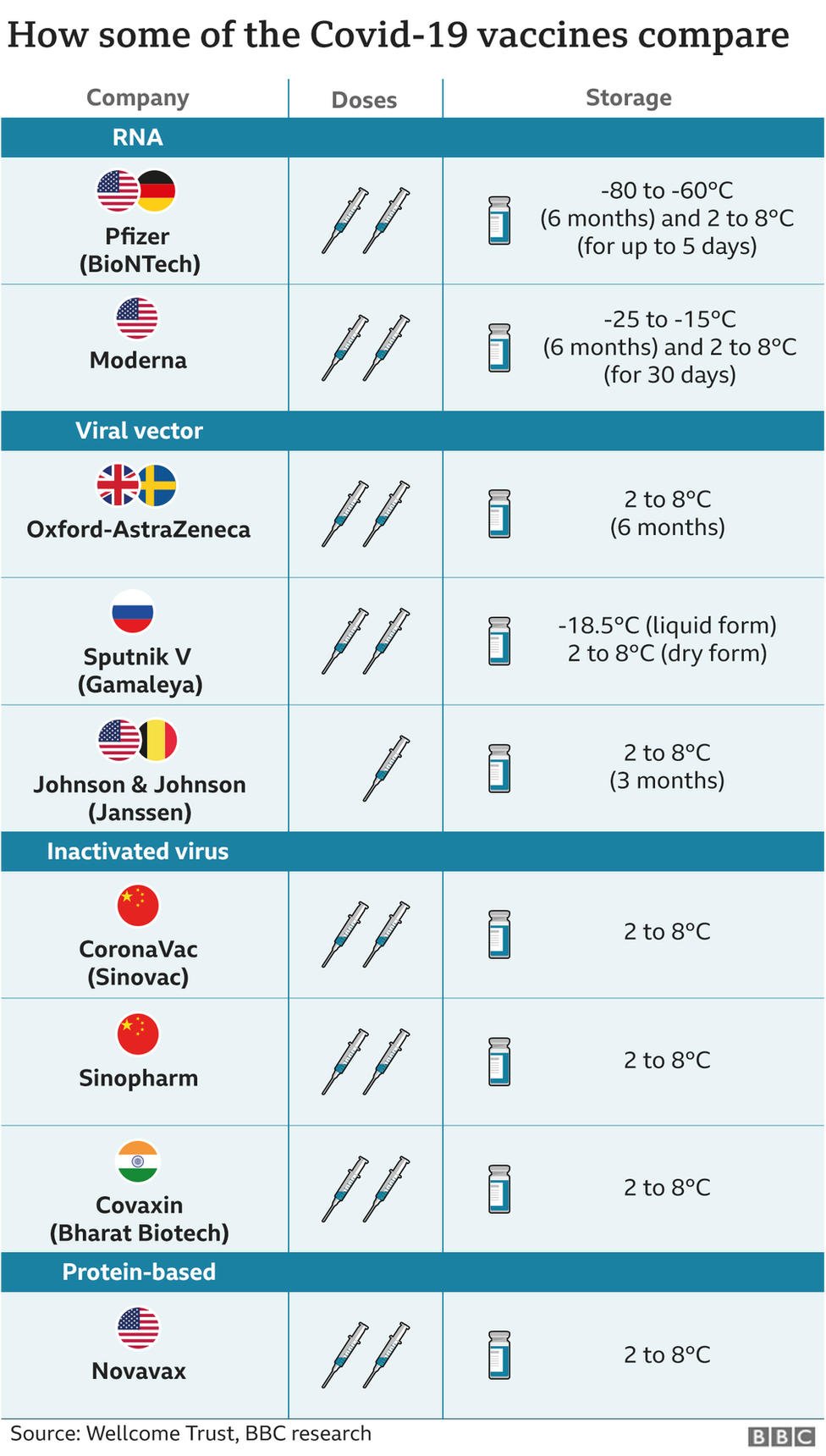
The Oxford-AstraZeneca vaccine, locally known as Covishield, is made from a weakened version of a common cold virus (known as an adenovirus) from chimpanzees. It has been modified to look more like coronavirus – although it can’t cause illness.
Covaxin by Indian firm Bharat Biotech is an inactivated vaccine which means that it is made up of killed coronaviruses, making it safe to be injected into the body.
The vaccine ran into controversy after India’s regulators gave it emergency approval in January while the third phase of the trial was still underway, sparking scepticism and questions from experts. Bharat Biotech, which makes the vaccine, has since published data suggesting 78% efficacy.
 IMAGE SOURCE,GETTY IMAGES
IMAGE SOURCE,GETTY IMAGESThe Sputnik vaccine, developed by Moscow’s Gamaleya Institute, also generated some controversy initially after being rolled out before the final trial data had been released. But scientists say its benefits have now been demonstrated.
It uses a cold-type virus, engineered to be harmless, as a carrier to deliver a small fragment of the coronavirus to the body. After being vaccinated, the body starts to produce antibodies especially tailored to the virus.
The ZyCoV-D vaccine uses plasmids – or small rings of DNA that contain genetic information – to deliver the jab between two layers of the skin.
The three-dose ZyCoV-D vaccine prevented symptomatic disease in 66% of those vaccinated, according to an interim study quoted by the vaccine maker Cadila Healthcare.
It is also India’s first needle-free Covid-19 jab – administered with a disposable needle-free injector, which uses a narrow stream of the fluid to penetrate the skin and deliver the jab to the proper tissue.
Previous DNA vaccines have worked well in animals but not humans.
The challenge, say scientists, was how to push the plasmid DNA into the human cell so that it gives a durable immune response.
Dr Jeremy Kamil, a virologist at Louisiana State University Health Sciences Center in Shreveport, told the BBC that it was imperative that the efficacy data of the vaccine “be vetted independently”.

Are there any other vaccine candidates?
The other candidates which are in different stages of trials in India to test safety and efficacy include:
- HGCO19, India’s first mRNA vaccine made by Pune-based Genova in collaboration with Seattle-based HDT Biotech Corporation, using bits of genetic code to cause an immune response
- A nasal vaccine by Bharat Biotech



No comments:
Post a Comment
Note: only a member of this blog may post a comment.